With a lack of historical population-based information to steer COVID-19 research, pharmaceutical companies are struggling to understand the everchanging virus as they work tirelessly to develop a vaccine in less than one year. Research teams can access near real-time COVID-19 patient data with Touchstone® for COVID-19 National Data Sets and Registry from over 80 million patients across the United States and three national data sources: John Hopkins University, The New York Times, and The COVID Tracking Project.
The Registry offers up-to-date, comprehensive data with outcome analysis and clinical trial analysis so research teams can stay up to date through every stage of the vaccine development process.
 Download
Download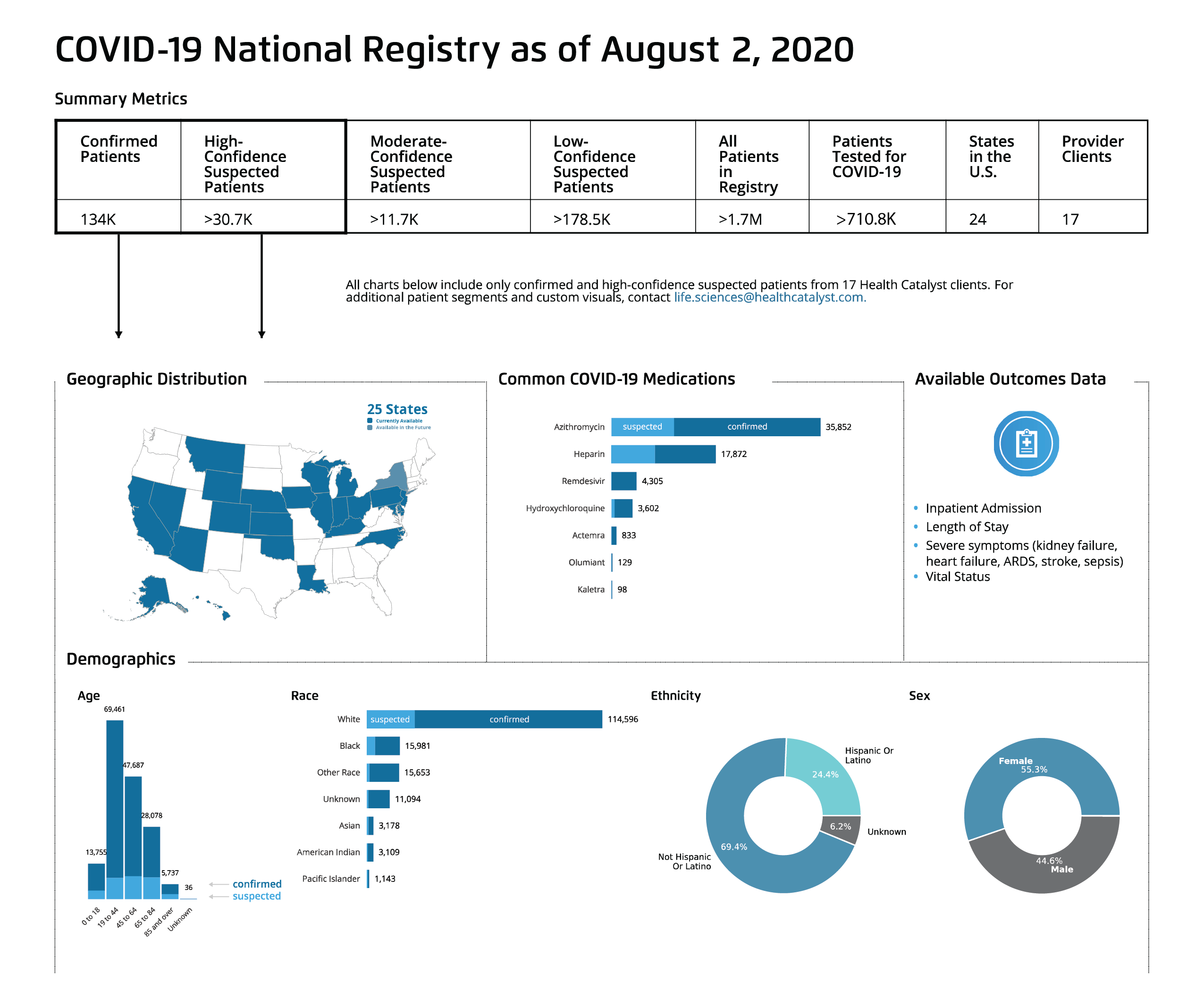

The World Economic Forum suggests the standard vaccination development process is 10 years. But, given the immense impact and transmissibility of COVID-19, pharmaceutical companies are working to accelerate the process and release a vaccine in less than one year. While the Food and Drug Administration (FDA) has rigorous safety and efficacy standards that pharmaceutical companies must meet before releasing a vaccine for public use, COVID-19 has forced a change in the rules in order to expedite the process.
With a shorter-than-normal time frame, research teams are under pressure to understand the novel coronavirus outbreak and develop a vaccine with little existing data or research. Pharmaceutical companies and health systems alike need the latest population-based research to keep up with the ever-changing nature of the virus as they develop a vaccine and care for patients.
In the past, pharmaceutical companies have relied on claims data as the main data source to develop effective treatments and medication. However, the static nature of claims data only gives a snapshot of a patient, failing to provide the continuing information that helps research and care teams keep up with the latest virus information.
The virus has also caused major setbacks for clinical trials, now making patients wait even longer to receive experimental treatment for other diseases. Hospitals have braced for an onslaught of critically ill patients, and researchers have had to shelve clinical trials for other illnesses, all in response to COVID-19. Agencies and clinical-trial funders have shown remarkable flexibility throughout the pandemic; however, research teams need access to near real-time data and insights from the frontlines to assess the long-term effects of COVID-19 on clinical trials and get back on track as soon as possible.
To overcome the lack of data and information about COVID-19, health systems are constantly tracking and recording new data about the virus. Now the primary source of information—de-identified COVID-19 patient data from health systems across the country—is an imperative to creating a vaccine and getting life back to normal.
Even though there is no historical COVID-19 patient data, pharmaceutical companies can still leverage comprehensive data for vaccine development and clinical trial processes. The Touchstone® for COVID-19 National Data Sets and Registry allows pharmaceutical companies to access de-identified patient data from more than 80 million patients across the United States (Figure 1), as well as three national data sources (Johns Hopkins University, The New York Times, andThe COVID Tracking Project). Now, pharmaceutical companies can tap into a breadth of data to conduct population-based research.

Partnering with Health Catalyst bridges gaps in health data with an ecosystem that spans hospitals, academic medical centers (AMCs), and research institutions across the United States. With unparalleled access to large sums of clinical data, coupled with support and expertise from Health Catalyst leaders to develop real-world insights and evidence, pharmaceutical companies can rely on added bench strength and up-to-date information from a comprehensive COVID-19 registry.
The Touchstone COVID-19 National Data Set and Registry goes beyond data, enabling research in unique ways:
Because knowledge about COVID-19 is continually evolving, the need for population-based research at a national scale is critical. The most effective population-based research includes the latest, point-of-care data that development teams can leverage to steer research and development.
Reliable, actionable insights—rather than static, outdated data—allows pharmaceutical organizations to maximize resources in clinical trials, shift direction as the virus changes, and avoid wasting precious resources.
The COVID-19 patient data and population-based research also benefits pharmaceutical companies primarily focused on clinical trials. For example, patients who contract the virus while participating in unrelated clinical trials could steer the outcomes of that trial to misleading endpoints. In this way, reliable, accurate insights can help clinical trials stay on track in geographic areas that are recovering from COVID-19 and progress to the planning phase of the COVID-19 framework (Prepare, Prevent, Recover, Plan).
COVID-19 also impacts the recruitment process for clinical trials. For example, a lack of understanding about the virus makes it difficult to recruit patients to test new medications that might react negatively with the virus. With access to a wealth of continuing point-of-care data sets across multiple populations, pharmaceutical companies will more quickly understand the virus as it evolves and take this information into account for existing and future clinical trials.
Additionally, COVID-19 research will reveal insights that open the door to future collaboration between health systems and pharmaceutical companies. As COVID-19 research and insights reveal clinical practice trends, research teams can use this information to connect with health systems after the pandemic to review trends and opportunities to improve care or develop new treatments.
COVID-19 presents seemingly endless unknowns to pharmaceutical companies, health systems, providers, and patients. These uncertainties can complicate an already complex environment and stall research efforts.
The Touchstone for COVID-19: National Data Sets and Registry offers near real-time patient data with outcomes analysis and clinical trials analysis, so research teams can stay up to date with, and adapt to, the virus. This comprehensive, population-based research data benefits pharmaceutical companies—both research-focused and clinical trial-focused—by providing de-identified patient data from more than 80 million patients throughout the United States, integrated with three national data sources.
The registry allows research pharmaceutical teams to focus on research and development of the COVID-19 vaccine and conducting clinical trials. With around-the-clock access to the most recent and complete data sets, pharmaceutical companies are prepared to battle the coronavirus with data-informed research and prepare for future healthcare unknowns.
Would you like to learn more about this topic? Here are some articles we suggest:
Would you like to use or share these concepts? Download the presentation highlighting the key main points.