Under a precision medicine approach, clinicians, academics, and pharma and biotech researchers and regulators aim to deliver the right drug for the right patient at the right time. Data, however, can present a challenge to precision medicine goals due to gaps in clinical care, research, and drug development when organizations don’t have the ability to capture and report on relevant real-world data. With the right systems to collect and share clinical and molecular data, the healthcare industry can realize the full benefits of precision medicine.
 Download
Download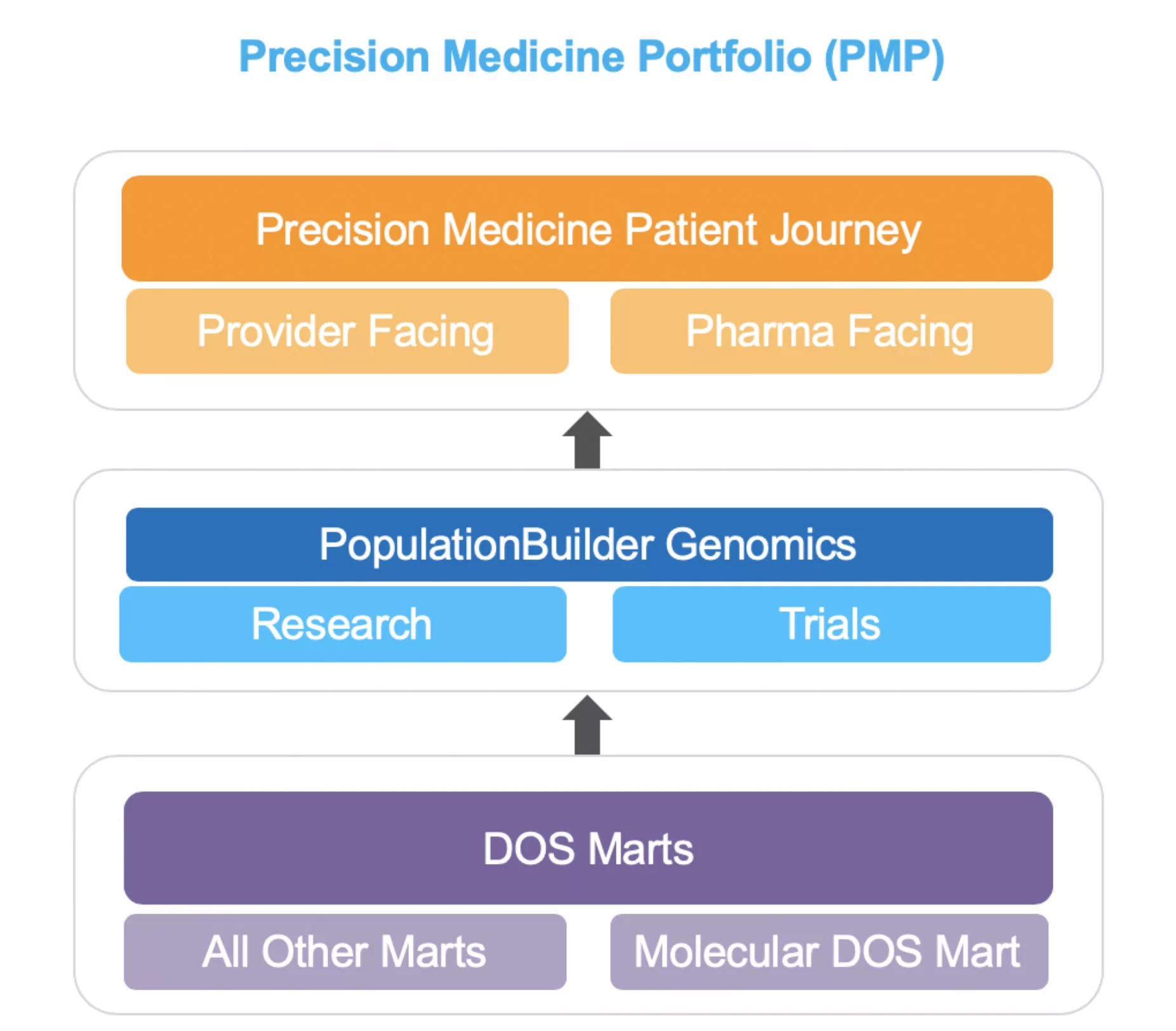

Healthcare is moving towards a highly individual model of precision medicine, which aims to deliver “the right drug for the right patient at the right time”—particularly for cancer treatment. With this individualized approach, targeted therapies are an increasingly prominent component of care.
For clinicians, academics, and pharma and biotech researchers and regulators, the biggest challenge in meeting precision medicine goals and optimally leveraging targeted therapy pertains to data—establishing the ability to collect and share data for patient care, research, drug development, and reimbursement. Capturing and reporting on relevant real-world data (RWD) is a top asset.
This report examines the gaps in clinical care, research, and drug development inherent with a lack of combined molecular and clinical data. It also considers how accessible data, products to use that data (e.g., the Health Catalyst® Precision Medicine Portfolio [PMP], Figure 1), and a core data management system (in this case, the Health Catalyst® Molecular and Clinical DOS Marts™ can help clinicians, academics, pharma, and biotech researchers and regulators advance the precision medicine goals of highly individualized treatment.
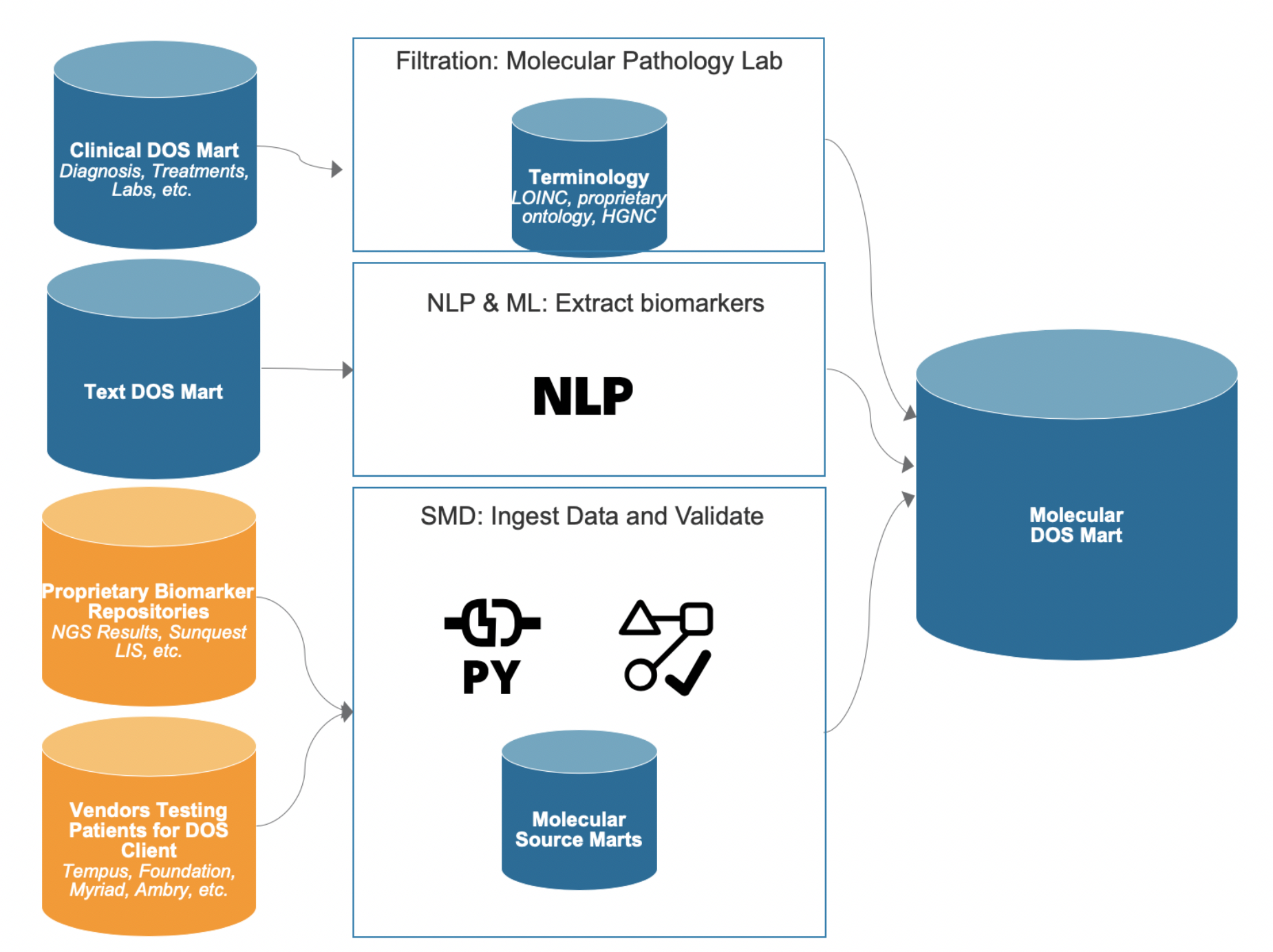
The PMP is a suite of products fully integrated into the Health Catalyst Data Operating System (DOS™) and includes applications that manage and analyze clinical, research, and operational data—including molecular data.” The Molecular DOS Mart (Figure 2), fundamental to the portfolio, is a data transformation and management model that incorporates data from disparate sources, including genomics, transcriptomics, and even molecular pathology labs (e.g., polymerase chain reaction [PCR] and ImmunoHistoChemistry [IHC] testing data). The extended Population Builder™: Stratification Module incorporates data from the Molecular DOS Mart to complement rich clinical data for cohort building and aggregate querying for researchers in academia, pharma, and biotech.
Research indicates that wait times vary between identification of a genetic mutation and receipt of the appropriate targeted therapy. This gap between molecular testing and targeted therapy raises the question of whether patients are receiving the timeliest treatment possible. By better understanding the interval between testing and prescription or administration and influential factors, health systems and life sciences organizations can move towards treating patients with care that uniquely meets their needs, with the most optimal timing.
For example, some patients might already be on a treatment plan (e.g., chemotherapy or radiation treatment) when they undergo molecular testing, which may account for the gap in receipt of the targeted therapy. Understanding treatment on this operational level helps care teams and researchers benchmark processes accordingly so they can link delays with certain outcomes and, in some cases, identify instances in which a longer delay would benefit the patient.
The Molecular DOS Mart uses a comprehensive schema, harmonizing molecular information from disparate sources, including lab testing such as PCR and IHC, as well as next-generation sequencing (NGS) testing. The Molecular DOS Mart includes patients’ molecular test data describing the following:
The above information is combined with the therapy order or administration and the associated date timestamp from the Clinical DOS Mart; together, they allow assessment of the number of days between molecular testing and order or administration of a targeted therapy for a given biomarker-drug association. This simplifies assessment of the number of days between molecular testing and order or administration of an associated targeted therapy by hospital, drug, and gene. The Clinical DOS Mart also enables filtering by diagnosis, which is relevant as more therapies become approved in additional indications.
Using Clinical and Molecular DOS Marts, the PMP contributes three critical assets to help life sciences organizations advance precision medicine goals:
Linked molecular and clinical data enables a view into the patient journey, called the patient longitudinal record. The PMP includes an application that surfaces an individual patient’s molecular test results and clinical information—including diagnoses, medications, and procedures, as needed—for clinicians and researchers alike. This information is rarely combined in one place, but by leveraging the Molecular and Clinical DOS Marts, Health Catalyst is uniquely positioned to provide a patient journey application. Such a tool can help clinicians interpret genomics data in the context of clinical and operational data within their workflow while also providing a researcher- and pharma-facing view for patients participating in clinical trials.
Leveraging both the Molecular and Clinical DOS Marts sourcing data from the point of care in real time or minimal lag enables researchers to answer additional questions surrounding precision medicine. Clinicians, academics, and pharma and biotech researchers can determine the number of patients on a targeted therapy who also received molecular testing prior to or following treatment. Furthermore, the Molecular and Clinical DOS Marts can answer broader questions about targeted therapies in the clinical setting—such as length of time between approval of a targeted therapy, efficacy of drugs, and overall adoption of the drug. The Molecular DOS Mart can provide clinicians and academic, pharma, and biotech researchers with longitudinal molecular data linked with diverse clinical data and outcomes, enabling biomarker identification and research.
Precision medicine clinical trial design must efficiently and dynamically incorporate genomic data and assess the value of matching profiled patients whose tumors harbor unique genomic alterations to specific clinical trials. These demands drive a market need for cohort design tools based on combined biomarker and clinical inclusion or exclusion criteria. With its broad-reaching access to RWD, the Health Catalyst PMP, powered by combined Molecular and Clinical DOS Mart, can serve as a singular and comprehensive source for patient identification.
Realizing the precision medicine goals of personalized care (treating the right patient with the right drug at the time) rests heavily on data. Academic medical centers, hospitals and life sciences companies that can have timely access to molecular and clinical data and a capable core data management system will be best positioned to deliver on the promise of individualized care and targeted therapies.
Would you like to learn more about this topic? Here are some articles we suggest:
Would you like to use or share these concepts? Download the presentation highlighting the key main points.