Comprehensive COVID-19 understanding is a critical asset for adapting to pandemic needs, directing resources, developing vaccines, and planning for surges in a timely, informed manner. Because common barriers have impeded the progress of comprehensive data repositories, researchers have relied on surveillance data from population-level viral testing, which has proven insufficient.
To significantly advance COVID-19 understanding, the medical community needs a digital patient registry that captures national-level data on how the virus impacts individuals differently according to comorbidities, lifestyle factors, and more. These essential insights lie in real-world evidence, which a registry can only deliver when it applies value sets to leverage clinical and claims data from health systems across the United States.
 Download
Download

Population-level viral testing for COVID-19 hasn’t produced the necessary level of surveillance data to inform efforts to contain the virus. The most significant challenges for building a digital national patient registry of COVID-19 patients range from variations in testing protocols, disparate and insufficient testing facilities, and differences in treatment strategies to data interoperability issues with multiple vendors, a lack of a singular data platform, data access and rights, and variations in local and state public health strategies, including surveillance.
To understand the virus comprehensively—how it impacts individuals differently according to comorbidities, lifestyle factors, and more—researchers need real-world evidence (RWE) for COVID-19 at an unprecedented speed. Digital national-level real-world data (RWD) registries represent an emerging solution for COVID-19 RWE and, therefore, a better understanding of the outbreak.
Soon, medical researchers will need COVID-19 RWE to understand the safety and efficacy of vaccines. For the newly approved vaccines that will run on expedited timelines, there will be insufficient or no data for children, older adults, adolescents, and underrepresented minorities. The clinicians, medical researchers, and policymakers will need ongoing data from the real-world setting to understand this disease, which affects the vast majority of the world.
To succeed in furthering COVID-19 understanding, patient registries must operate on a minimum viable data set (MVDS). These data sets comprise elements forming the essential data that hospitals, testing facilities, and public agencies would collect for patients who have COVID-19 symptoms but haven’t tested positive for the virus and patients testing positive for COVID-19. The clinical and scientific community, along with policymakers, use the MVDS to build registries and leverage testing, clinical, and claims data from health systems across the United States, creating cohorts for confirmed and suspected COVID-19 patients.
No singular national authority sets MVDS requirements for COVID-19. As a result, several registries have emerged that lack standardization and cover limited breadth and depth of the data necessary to answer critical questions about COVID-19 for the medical research community and policymakers.
These insufficient existing COVID-19 registries are confronting the global pandemic’s pressure on the worldwide community the think holistically about the healthcare and life sciences ecosystem and the collection and use the data to support various use cases. Hospitals serving COVID-19 patients, as well as medical researchers and pharmaceutical companies, face the following challenges responding to the outbreak:
With the pandemic driving the above challenges and an urgent need for collaboration and new actionable data, as well as understanding what post-pandemic healthcare and life would look like, digital registries are well-positioned to support aggregated, RWD collection. Until medical researchers publish the results of COVID-19-related randomized control trials and healthcare eradicates COVID-19 or has scientifically proven treatment options. With the FDA's support of patient-generated RWD and RWE, healthcare and life sciences organizations must leverage the data available through disparate data sources including EHRs and claims to improve decision making around COVID-19 in clinical, research, and policy settings.
The pandemic has necessitated expanded uses of RWE to support regulatory decisions. By conducting RWE studies that apply the appropriate scientific standards to the COVID-19 registry and following use cases, life sciences can support public health and policymaking by ensuring the safety and efficacy of interventions—for COVID-19 and beyond.
An MVDS approach fills in the information gaps by leveraging clinical and claims data from health systems across the United States, powering a dynamic multipurpose COVID-19 national patient registry and RWE related to COVID-19. One such registry solution from Health Catalyst, the Touchstone® COVID-19 Registry and Insights (Figure 1), aims to provide a template for other broader national registries for COVID-19 and beyond.
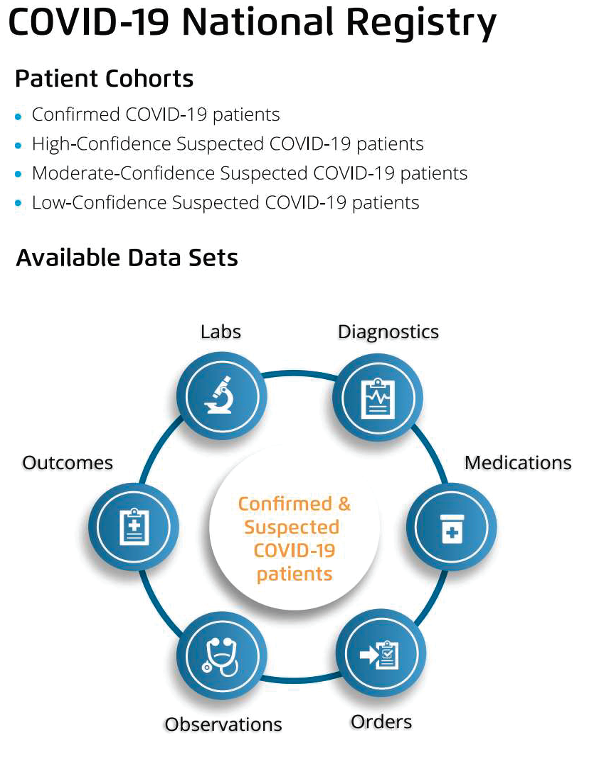
A national-level registry effectively assembles critical data in a standardized manner, giving healthcare and life sciences organizations a documented, reproducible solution to the pandemic data scarcity and quality challenges. Concepts added in this registry can serve as an MVDS enables the exchange of essential data and improves the quality of data collected, which is vital as disparate sources and organizations collect the data. Also, users can configure their EHRs and other front end data capture systems to manage MVDS, making it an accessible tool for many organizations.
By leveraging Health Catalyst COVID-19 national patent registry to identify key characteristics of interest that define COVID-19 patients, it is easier to track trends over time as patients progress from suspected to confirmed diagnoses. Tracking serves several benefits, including assisting with triaging of patients to perform appropriate billing of ICD-10 codes and predicting and triaging patients with COVID-19 signs and symptoms who are more likely to test positive. Meanwhile, the documentation and coding standards for COVID-19 suspected and confirmed cases are evolving, and further assessments and studies will help fully leverage the registry in COVID-19 efforts.
The digital COVID-19 registry allows for two essential capabilities to further understand the virus: defining patient cohorts and lab test knowledge curation (Figure 2).
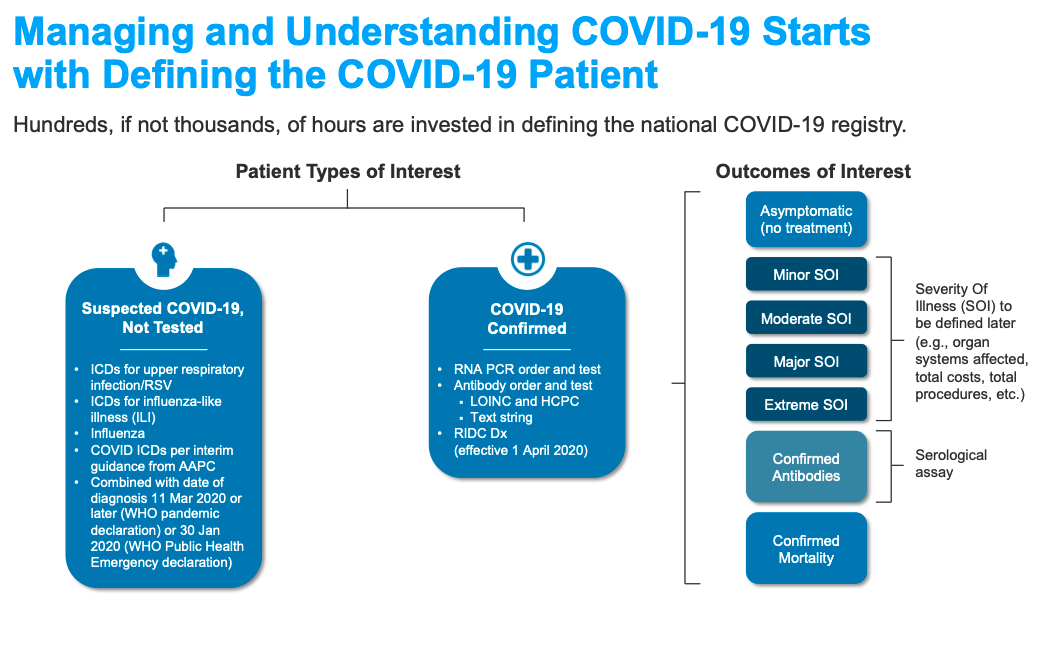
Based on guidance from national and global agencies, including the Centers for Disease Control and Prevention, World Health Organization, and others, proposed value sets must include two patient cohorts for the registry—confirmed and suspected COVID-19 patients. The guidelines further stratify the cohorts into three levels of confidence (Figure 3):

The patient cohorts for the registry defined using the value sets above allow the study of patient care outcomes. Examples of outcomes of interest in COVID-19 patients include the following measures:
Due in part to the critical diagnostic importance of lab testing, lab test result data is vital to understanding patients’ clinical state and surveillance of patient populations. However, it has been extremely challenging for the clinical, research, and public health communities to get lab data from hospital and reference labs with consistent LOINC codes and manufacture information.
EHR systems often store lab data with local codes for test types and result values rather than codes from widely standardized terminologies (e.g., LOINC (Logical Observation Identifiers Names and Codes)). In such cases, researchers need to ascertain the types of lab tests and the meanings of the result values automatically over a large volume of lab result data—without the benefit of a uniform standard lab terminology across the various EHR systems the proved the lab result data.
In the Health Catalyst national-level COVID-19 registry, a lab test knowledge curation workflow provides a knowledge base for recognizing lab test types and understanding lab result values, as expressed in the lab result data records from multiple EHRs (Figure 4).
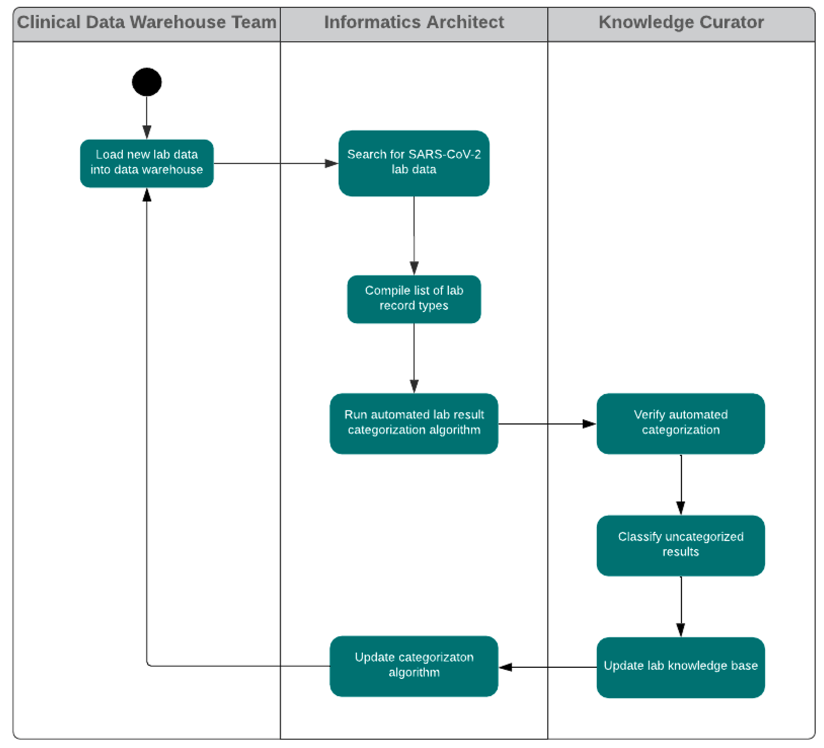
The lab test knowledgebase, accumulated from many lab result records across multiple EHRs, allows for automated categorization of lab results (e.g., positive, negative, pending, ambiguous, test problem, unmapped) and lab test types (e.g., detection of COVID-19 material or a COVID-19 antibody). This classification system identifies patterns of interest; for example, it classifies the data of patients who are confirmed or suspected COVID-19 cases. The harmonized set of lab result values allows for the following:
Manufacturer information integrated with lab test orders, results, and clinical data can help assess various COVID-19 diagnostic tests’ performance. An EHR, Lab Information System, and the instrument collect several data points. No system captures the full picture, as different providers and systems are involved. There are interoperability challenges and a lack of recommended MVDS to enable standardized data collection and curation efforts to support RWE.
To address this industry-wide challenge, Health Catalyst is actively addressing this problem in a collaborative approach with health systems and diagnostic labs to add the data elements of interest from unstructured and structured data in EHRs and Laboratory Information Management Systems. This will enable RWD in the COVID-19 registry to understand the performance and the limitation of testing and understand better how to use data for RWE.
While the COVID-19 registry holds promise in better disease understanding and management, optimizing it requires more work with healthcare and life sciences organizations of every size to help their analytics and informatics team develop a registry. The participation of EHR vendors is also crucial to ensure they can leverage their partnerships with hospitals for maximal data collection. Additionally, federal government oversight and leadership by coordinating with local health authorities would coordinate the collection of adequate data to direct current and future decision making to control this pandemic and prevent subsequent outbreaks.
To generate evidence and insights from this registry and other data sets at a national scale, the Health Catalyst Life Sciences division is participating in the COVID-19 Evidence Accelerator launched by the Friends of Cancer Research and the Reagan-Udall Foundation for the FDA. Health Catalyst has shared experience with the COVID-19 national patient registry with the Reagan-Udall accelerator to inform the key data elements needed to support RWE generation for COVID-19.
Understanding the importance of partnerships with several national and local authorities, Health Catalyst collaborated with the MITRE Corporation to convene the COVID-19 Healthcare Coalition. This group of EHR companies, non-profits, analytics companies, healthcare organizations, and academic community members contributes knowledge to solve public health questions related to COVID-19. The coalition has defined common data elements and study designs to allow its members to replicate these investigations with each of their patient populations independently; no member working alone could produce these insights.
As a dynamic and scalable approach, leveraging EHR data with MVDS can accommodate the rapidly changing COVID-19 landscape and fill in critical gaps of population-level viral testing. Aggregated outbreak information from health systems across the United States will give researchers and providers the comprehensive understanding they need to adapt to pandemic demands, direct resources, and plan for surges in a timely, informed manner.
The Touchstone COVID-19 Registry and Insights work represent a historic collaboration between EHR and analytics vendors. The complete study is available on the COVID-19 Healthcare Coalition’s website, courtesy of MITRE.
Would you like to learn more about this topic? Here are some articles we suggest:
Would you like to use or share these concepts? Download the presentation highlighting the key main points.
Click Here to Download the Slides
https://www.slideshare.net/healthcatalyst1/the-fight-against-covid19-a-national-patient-registry