When realized, the promise of precision medicine (to specifically tailor treatment to each individual) stands to transform healthcare for the better by delivering more effective, appropriate care. To date, to achieve precision medicine, health systems have faced financial, data management, and interoperability barriers. Current trends in healthcare, however, will give researchers and clinicians the quality and breadth of health data, biological information, and technical sophistication to overcome the challenges to achieving precision medicine.
Four notable trends in healthcare will bolster to growth of precision medicine in the coming years:
1. Decision support methods harness the power of the human genome.
2. Healthcare leverages big data analytics and machine learning.
3. Reimbursement methods incentivize health systems to keep patients well.
4. Emerging tools enable more data, more interoperability.



Precision medicine stands to deeply transform healthcare by tailoring care precisely to the individual, taking into account such personal characteristics as ethnicity, local environmental exposures, immunization history, and more. This exacting approach to care will leverage big data, genomics, and increasing interoperability to help clinicians identify the most appropriate, cost-effective treatments, and even spot previously undetected causes of disease.
This report will define precision medicine (explaining how it differs from population-based medicine), discuss real-world benefits of precision medicine, and look at how greater biological insight (the human genome) and novel technologies can help overcome the financial, data management, and interoperability barriers to fully realizing the promise of precision medicine.
President Obama announced the Precision Medicine Initiative in his 2015 State of the Union address. Under the initiative, medical care would transition from a one-size-fits-all approach to an individualized approach, in which data on each patient’s genomic makeup, environment, and lifestyle (the exposome) helps medical professionals tailor treatment and prevention strategies. To achieve the Precision Medicine Initiative mission statement, “to enable a new era of medicine through research, technology, and policies that empower patients, researchers, and providers to work together toward development of individualized care,” researchers and clinicians need vast and varied amounts of data and the technology to ensure that data is widely accessible and usable.
The healthcare industry often uses the term “personalized medicine” to describe a population-based approach to care; personalized medicine is less individualized than precision medicine. Personalized medicine is a one-size-fits-all approach to healthcare, in which clinicians choose treatment according to protocols based on cohorts of patients that may not represent all individuals.
In reality, healthcare is progressing along a continuum from population health to personalized medicine to precision medicine. The goal at the precision level (to improve health and treat disease by tailoring treatment to individuals) picks up where population and personalized approaches leave off.
For example, a health system will treat a 60-year-old woman with diabetes using the protocol shown effective for 60-year-old women with diabetes. This population-level guideline, however, doesn’t take into account other medical and lifestyle factors affecting the patient’s health and ability to follow the treatment plan, such as comorbidities and physical limitations or environmental factors that make self-care or accessing medication and supplies difficult. Likewise, the cohort population used to develop the treatment guideline may not be consistent with the patient’s ethnicity and environment.
In more specific, real-life examples, a non-precision approach to care can mean insurance companies base medication coverage on population-based guidelines, rather than what’s appropriate for a specific patient, or that clinicians choose inappropriate treatments with dangerous risk side effects:
As appealing as the promise of individually tailored treatments for each patient is, researchers and innovators face significant challenges in achieving precision medicine. With the support of several prominent healthcare trends, however, the champions of precision medicine will have the capability to access and manage the vast amount of data needed to drive individualized care while improving outcomes and lowering costs.
Given four notable trends in healthcare, precision medicine stands to grow and transform the way patients experience care over the next few years:
Precision medicine uses genomic information (in addition to medical histories and patients’ self-reported experiences) to determine the best care for each individual. The National Human Genome Research Institute (NHGRI) defines genomic medicine as “an emerging medical discipline that involves using genomic information about an individual as part of their clinical care (e.g., for diagnostic or therapeutic decision-making) and the other implications of that clinical use” A human genome contains an individual’s complete set of DNA, which includes all genes.
Francis Collins, the former director of NHGRI, described the genome as a book with multiple uses and “a transformative textbook of medicine, with insights that will give healthcare providers immense new powers to treat, prevent, and cure disease.” In the care setting, these insights can help clinicians make treatment and prevention decisions that target an individual’s actual risks and circumstances, versus following population-based guidelines that may not serve that patient.
For example, according to a 2009 study published in The New England Journal of Medicine, for men over 50 in the general population prostate specific antigen (PSA) testing hasn’t significantly reduced morbidity and mortality over the past decade. While annual PSA testing may not be appropriate for all men, some men may be at high risk for death from prostate cancer, which genomic profiling can indicate. Regardless of population-based guidelines, these high-risk men may still benefit from annual prostate testing (by catching prostate cancer in its early, most treatable stages).
In precision medicine, researchers and clinicians who aggregate the most genomes, exposomes, and rich patient clinical histories with advanced machine learning technologies, will have the best outcomes. However, tailoring care to more effectively treat and prevent diseases, requires significantly more robust data than clinicians and researchers currently collect and hold in the EHR. Specifically missing is patient-generated data. They’ll also need analytical tools (e.g., machine learning) that can turn this vast amount of complex data into insights about care.
The previous prostate risk example shows how precision medicine requires machine learning (versus generalized analytics), as genomic information makes disease identification less deterministic. Instead of looking at columns and rows to identify disease, users will have to work with probabilities. The genomic factor, with the support of machine learning analysis to see beyond traditional data sets, gives a more specific picture of an individual’s health. For example, if the prevalence of prostate cancer is 5 percent, and an individual has an increased risk of 50 percent, his increased risk is 7.5 percent. Due to this increased risk, this patient should receive a PSA test, although he generally falls into the population-based cohort that would preclude testing at his age.
As evidence of the current value of genomics, major healthcare organizations are acquiring startups in the field. Recently, Centene, the largest Medicaid processor in our country, acquired a clinical and genomics data analytics business, company Interpreta. With Interpreta’s genomic information, Centene, gains the necessary insight to deliver precise care at the right time and at the right cost for the under-insured and uninsured.
With the more complex nature of genomic data (compared with relatively simple data points, such as lists and lab results) clinicians need more sophisticated technologies to access data. Existing EHRs are limited in their data-sharing abilities (and their genomic content), but new ways of exchanging data, such as FHIR-based APIs that can ingest medical records from major EHRs, will enable the interoperability precision medicine requires.
With FHIR now in every iPhone and the growing availability of mobile apps and wearable devices that can detect everything from blood sugar levels to sleep patterns, patients are increasingly transmitting data from the home. This level of interoperability adds to the data set by enabling patient-reported outcomes and subjective information (e.g., mood, pain levels, etc.).
With this clear demand for greater data sharing, the Argonaut Project is addressing challenges to modern, open interoperability standards. This impacts precision medicine as data-sharing and interoperability standards between EHR vendors must expand to allow for individually tailored care. The Argonaut project creates an ecosystem of apps that allow EHR vendors to connect with the project’s set of clinical decision-support tools. All major EHR vendors have committed to adopting the Argonaut standards in 2018.
For example, Interpreta, a cloud-hosted, genomics-interpretation service, works with the Argonaut Project’s standards to help provide patients with accurate and timely care (a decision-support service). A health system EHR can contact Interpreta, which evaluates a patient’s chart against the genomic information in its database and develops a treatment recommendation. In a mature precision-medicine environment, these sorts of decision-support tools will be plug-ins that will run in the background on the EHR. Frontline clinicians won’t even be aware of them.
BIDMC@Home, Beth Israel Deaconess Medical Center’s home-monitoring app (Figure 1) translates between the data a patient collects at home and the clinician’s office, ensuring that information is accurate. BIDMC@Home uses a care plan creator, a tool within the EHR, to develop a daily care plan (including medications, diet, and activity) for each patient based on her health record and delivers that plan to the patient’s phone.
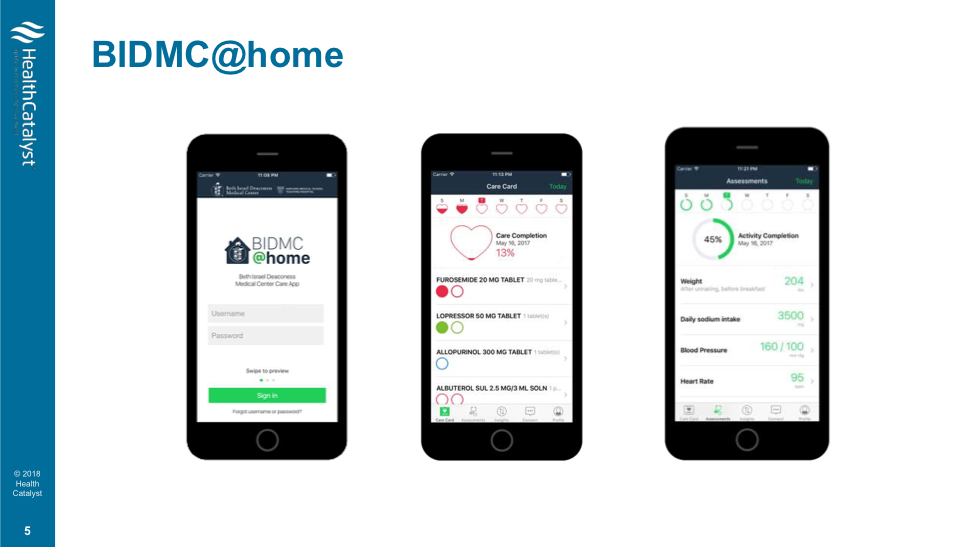
As payment-reimbursement models change from fee-for-service to value-based purchasing, precision medicine stands to be a cost-effective solution. Precision approaches aim to cut down on the redundant and unnecessary testing and avoidable hospital admissions, which drive up cost in a value-based setting.
This shift in reimbursement incentivizes health systems to take the types of actions that create the data precision medicine requires, such as putting bathroom scales in the home of patients with congestive heart failure and engaging visiting nurses, home care, telecare, and telemedicine. These interventions generate more data that helps keep individuals healthy and avoids hospitalization or unnecessary imaging, while building on the data loop that drives precision medicine.
Precision medicine helps close the health data loop by sustaining a continuous cycle of feedback. The loop builds a comprehensive health knowledgebase of clinical, self-reported, and biometric data (e.g., heart rates, activity levels, and sleeping habits) that helps clinicians and researchers better treat and prevent disease.
But to close this feedback loop of health data, simplifying operations — which in turn frees up resources to analyze the influx of new patient data — requires that the medical industry incorporate new skillsets. Stanford’s Department of Biomedical Data Science (DBDS) is trying to do just that: teaching doctors and researchers how to utilize medical data in practical ways.
According to the 2017 Gartner Hype Cycle for Emerging Technologies (Figure 2), blockchain is among the technologies forecasted to reach the peak of inflated expectation in 2018. But for precision medicine, blockchain may have real potential.
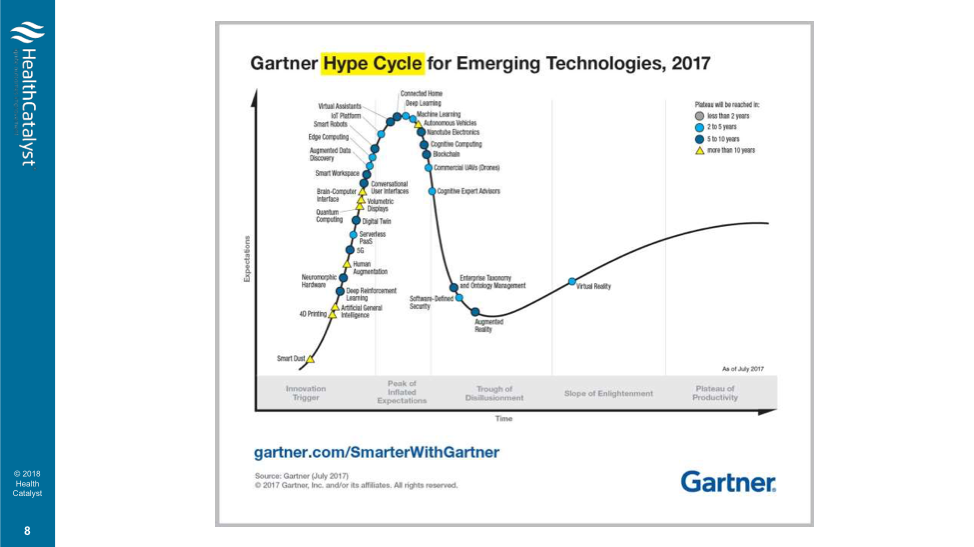
Because machine learning techniques are more powerful with bigger training sets and more data, precision medicine needs methods to get more data. Some researchers are proposing using blockchain to pay patients for their clinical data, internet-of-things data, or genomic data. The contribution, which could be anonymous, contributes to the larger body of health and genomic data needed for precision medicine.
Blockchain also functions as a good auditing tool and may have a role in care coordination. Today, about 25 percent of patients seek care outside of their network. Even with advanced interoperability, it’s difficult to track all the places an individual receives care and where his records are.
Healthcare blockchain users, however, have to take precautions when it comes to patient privacy. As a public ledger, blockchain discloses where a patient has sought treatment and, in doing so, discloses a possible clinical condition (e.g., if a patient has a record at an alcohol treatment facility, it suggests an alcohol abuse problem). A blockchain pilot program with Harvard and MIT is testing a consent management contract that protects privacy along with data integrity.
With this timely convergence of expanding genomic data, novel big data and machine learning technologies, new reimbursement methods, and potential tools (e.g., blockchain) on the horizon, precision medicine will have the infrastructure to realize its promise of providing exactly the care a patient might—or might not—need according to her personal genomic make-up and health history. To be a part of the transformation to truly individualized care, health systems will need to harness the potential of big data and machine learning capabilities to expand their data access and interoperability beyond traditional EHRs (including integrating genomic data), continue to adapt to value-based reimbursement, and stay aware of emerging tools that can individualize care and further the mission of precision medicine.
Would you like to learn more about this topic? Here are some articles we suggest:
Would you like to use or share these concepts? Download this presentation highlighting the key main points.