The life science industry has historically relied on sanitized clinical trials and commoditized data sources (largely claims) to inform its drug development process—an under-substantiated approach that didn’t reflect how a new drug would affect broader patient populations. In an effort to gain more accurate insight into the patient experience and bring drugs to market more efficiently and safely, the industry is now expanding into extended real-world data (RWD).
To access the needed breadth and depth of patient-centric data, life science companies must partner with a healthcare transformation company that has three key qualities:
1. A broad and deep data asset.
2. Extensive provider partnerships.
3. An outcomes-improvement engine to support the next generation of drug development.



The life science industry (pharmaceutical, medical device, biotech, digital therapeutics companies, and other innovators) has invested significantly in data—specifically, extended real-world data (RWD)/real-world evidence (RWE). More importantly, the industry has realized that focusing on population health management (PHM) and outcomes improvement is its guiding principle and top goal, and data is one part of how it will achieve that goal.
Patient-centric data beyond commoditized claims data assets is becoming instrumental in the drug development pipeline. It’s informing the process from discovery, new indications, clinical development, trial design, and measuring outcomes (e.g., side effects) to identifying who is using an approved drug and why and determining value-effectiveness for drug reimbursement. As data has become a staple decision driver at most life science companies, organizations are increasingly aware of the need to bridge the divide between these two data imperatives:
This report explains the importance of extended RWD and RWE for the life science industry and how partnering with the right healthcare transformation company provides access to the patient-centric data needed to improve the drug development process.
The drug development process takes from 8 to 15 years, costs up to $11 billion, and relies on an expensive and often inefficient clinical trials process, as well as costly, sparse data (often only claims data) that doesn’t provide a full picture of patient health. For example, claims data will show that a patient fills a prescription but gives no insight into outcomes, side effects, etc.
Life science companies can improve this process and save costs by partnering with a healthcare transformation company to access and leverage extended RWD and RWE to better understand the populations using their drugs and their outcomes. As the entire system (regulators, payers, manufacturers, and providers) aligns around outcomes, the following knowledge areas will enable a fair, outcomes-driven healthcare system:
Healthcare today has a crucial opportunity, as, for the first time, key industry players are aligning on the same key goals. Regulatory, cost, and reimbursement pressures are driving the urgency to deliver the right treatment to the right patient, as measured by real-world outcomes and monitoring. This means that manufacturers, payers, and providers all benefit from solving similar challenges:
While RWD (mostly claims and some EHR data) has shown more useful potential for life sciences in the past few years, its impact has often been limited to specific settings, disease areas, geographies, payers, etc. The coming years will likely see a substantial impact across most therapeutics, both for their development, launch, and post-launch activities.
The 21st Century Cures Act was signed into law in 2016, boosting the value of RWD and RWE for the FDA and life science industry. The Cures Act aims to accelerate medical product development and innovation and places more focus on RWE- and RWD-driven decision making. According to Congress, RWE is data from sources other than clinical trials (e.g., randomized trials and observational studies) on the use and the potential benefits or risks of a drug. Congress describes RWD as data on patient health status and/or delivery of care that’s routinely collected from EHRs, claims and billing, patient-generated data, and more. Both RWE and RWD are growing in volume and depth with the increasing use of computers, mobile devices, and wearables and gaining utility as advanced analytics capabilities (e.g., AI and machine learning) enable more personalized and actionable insights.
In 2018, the FDA published the Framework for FDA’s Real-World Evidence Program, which further details the usefulness of RWD in trials for new therapies. The emphasis on RWD is leading to a new approach that includes pragmatic trials (e.g., trials where the control arm is based on RWD from the standard of care) and synthetic cohorts (generating historical controls from historically accumulated trial controls, and/or simulating them on current RWD cohorts). Because pragmatic trials are poised to slash costs and reduce timelines drastically, life science companies that adopt them early will differentiate themselves in the market.
Key RWD/RWE challenges are emerging around access to health systems and patients as different organizations compete to enroll patients in traditional studies, as well as innovative studies that leverage data-driven approaches. It’s not sufficient for life science companies to leverage data; they must also create clear value for providers and patients, ensuring that innovations in clinical development help health systems achieve certain goals:
For much of the past decades, inefficiencies in clinical drug development amounted not only to money the pharmaceutical industry spent (from research to launch) but also in poorer overall outcomes for patients, who experienced a rigid, synthetic clinical trial environment (i.e., being put on a placebo arm for the sake of the trial design rather than the sake of the patient or excluded from promising trials due to the complexity of trial designs). A real-world approach marries clinical development with the realities of healthcare:
By leveraging extended RWD/RWE, life science companies gain critical value across their pipeline (Figure 1), allowing them to:

Offerings have started to grow around certain therapeutic areas (e.g., oncology EHRs), and healthcare analytics vendors are now expanding offerings to meet the demand for integrated data from many sources (e.g., labs, consumer behavior, and more [Figure 2]) that capture the breadth of patient health.
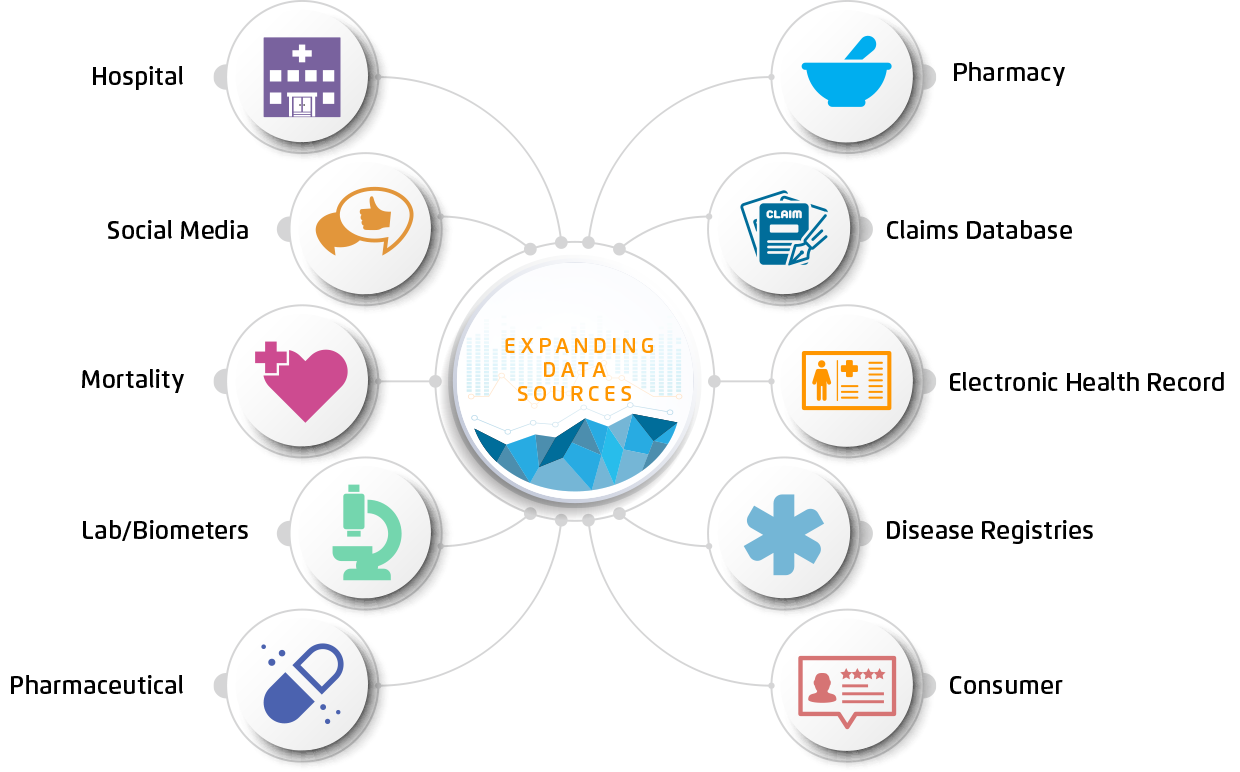
The life science industry historically placed its largest bets on heavily sanitized clinical trials, as regulatory approval equated to reimbursement. As the field evolved, developers learned that the synthetic trial setting didn’t reflect drug performance in the real world. When commercialized and available to larger populations, drugs may produce different safety profiles and effectiveness than in the select trial population.
The limited populations and controlled nature of clinical trials have sparked the extended RWD/RWE movement to understand patient behavior and real-world performance via data outside the clinical trial setting. And as regulators and payers adapt, the pressures have mounted to develop therapies that are safe and effective in the real-world setting.
Much of the initial evidence, however, was confined to large, but shallow, claims-derived datasets. These datasets present many challenges in the clinical trials setting:
A few deeper, EHR-derived datasets emerged, especially for some diseases (e.g., cancer) from specialized companies or from specific regions, payers, etc. Most of these datasets, however, are not broad and deep enough and can often only be partially linked together.
Driving outcomes improvement requires integration of data across sources, but with much of RWD to date focused on claims and with limited EHR data, the life science industry lacks the breadth and depth to leverage RWD in drug development. As Figure 3 explains, and the experience of healthcare transformation companies confirms, only 8 percent of the needed data resides in the EHR. Having large claims data and some EHR data is only scratching the surface of true outcomes measurement and transformation work for both population health and personalized approaches.
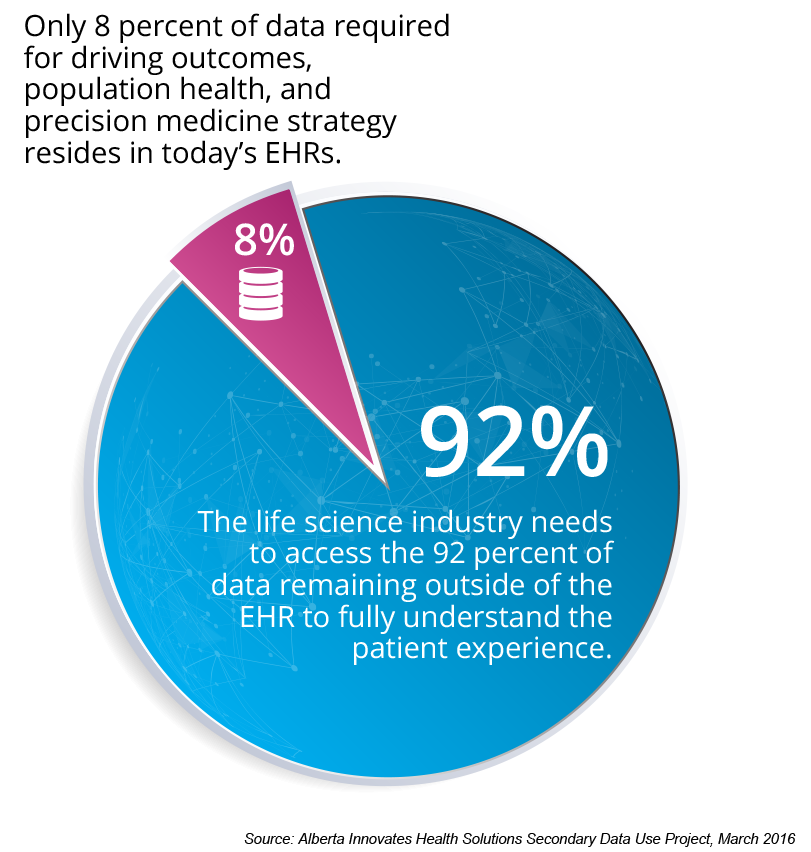
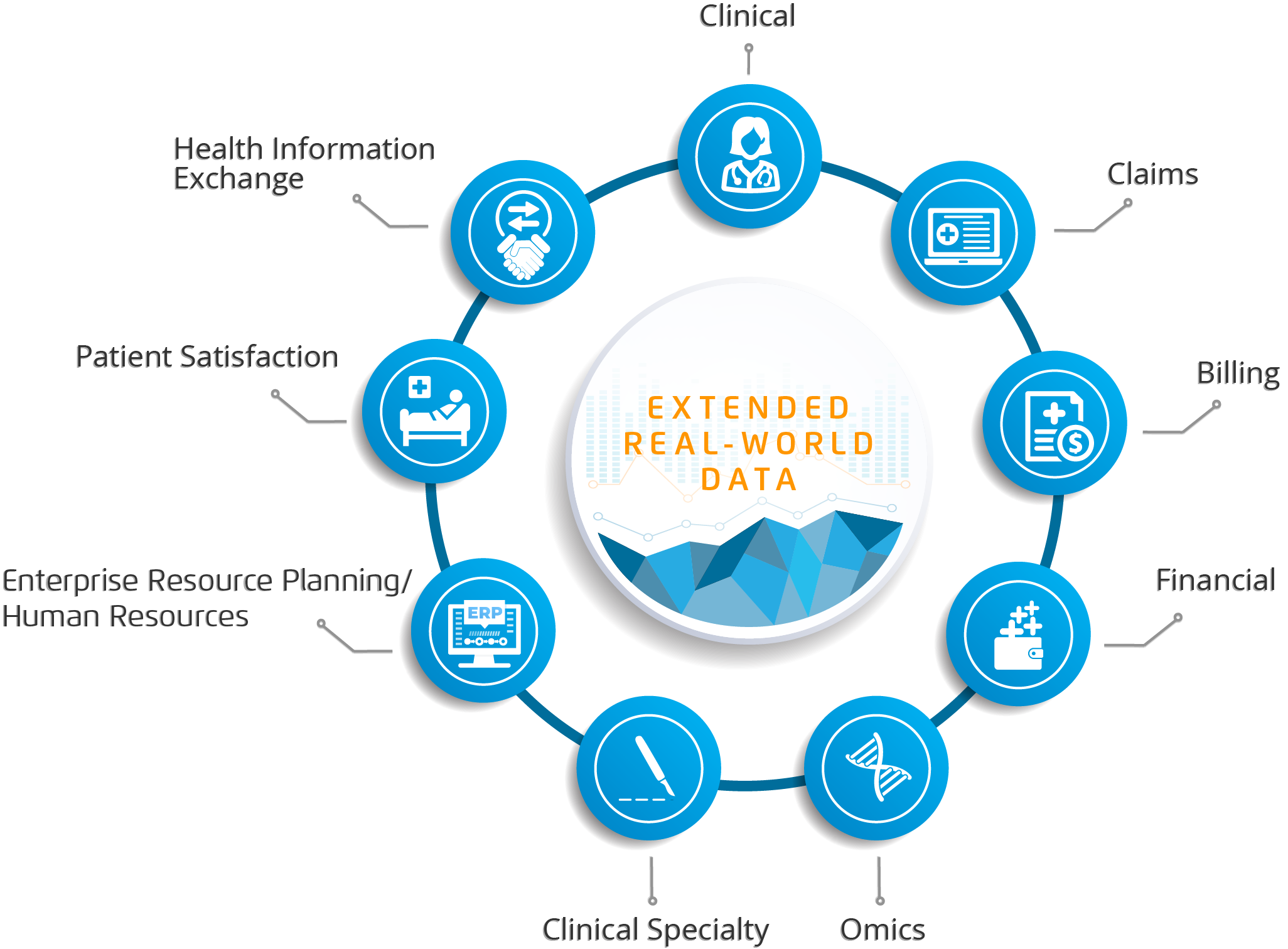
To fully understand the patient experience, life science needs to access the remaining 92 percent of data that resides in other systems. Specialty EHRs (e.g., oncology and cardiology) fill in some commercialized data gaps but miss a lot of the data that drives population health (e.g., cost, patient satisfaction, lab results, etc.). Extended RWD/RWE requires broader sources to truly understand patients; life science companies can access this knowledge by partnering with an established healthcare transformation company. Health Catalyst, for example, has more than 200 data sources integrated within its systems. As data sources expand into patient-reported information (e.g., from patient-facing apps), integrated vendor analytics will become more critical to round out extended RWD (Figure 4).
To produce extended RWD, a healthcare transformation company must have five key capabilities:
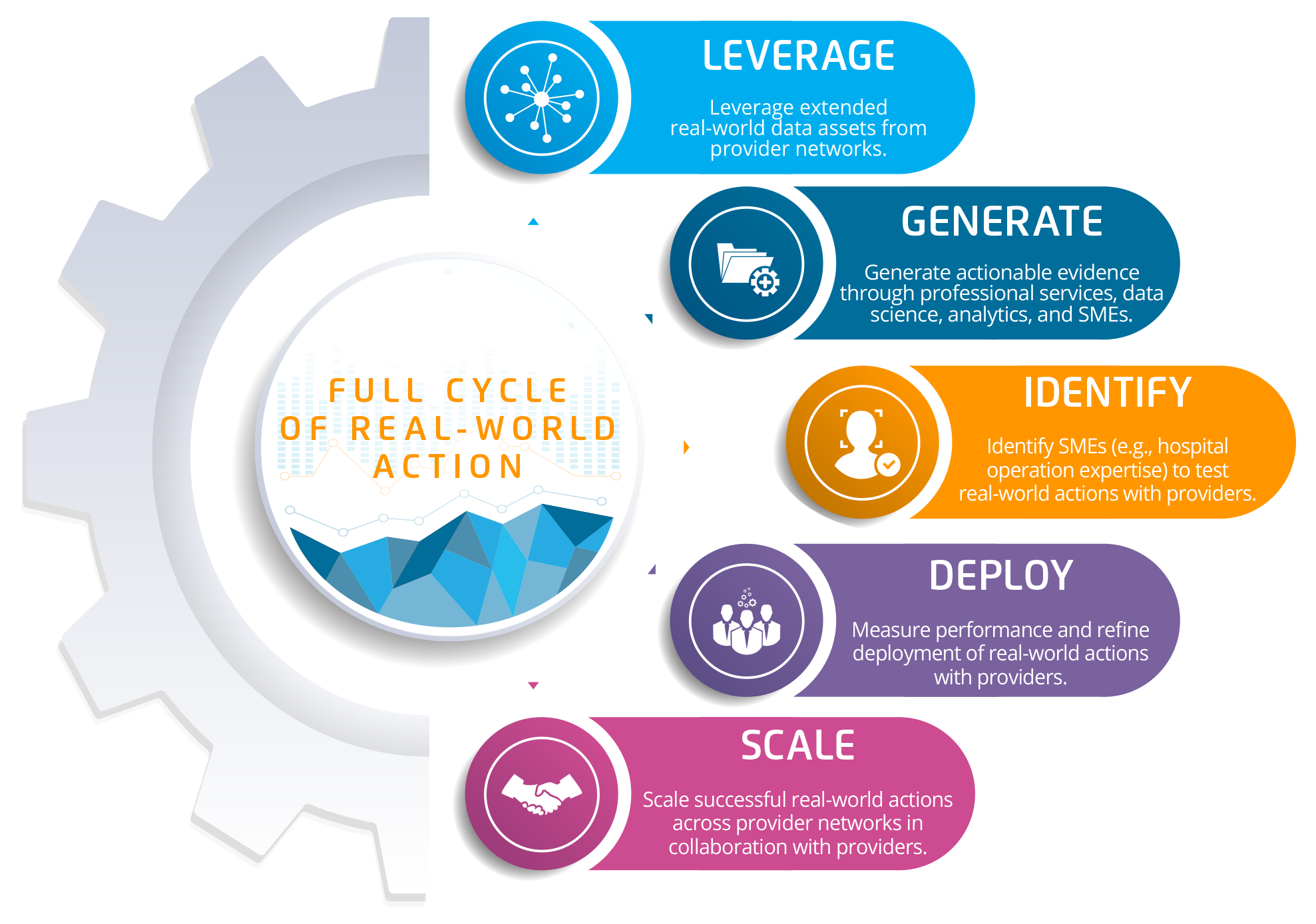
Having RWD and RWE is only the start to driving outcomes; life science companies need the ability to act on the data by turning it into provider-level actions that benefit the patient (e.g., running a clinical study, a patient education/engagement program, an adherence program, a safety program, etc.). Partnering with the right healthcare transformation organization helps life sciences achieve real-world action by connecting companies with multiple health systems and patients, as well as the data that determines whether hypotheses are operational and lead to improvement. For example, a life science company can use shallow data to predict drug response, find patterns, and develop insights, but only when it deploys the drug in the real-world hospital setting can it understand the actual real-world impact.
Together, the life science industry and the right healthcare transformation companies can drive change, monitor, and measure drug performance. This completes the full circle of real-world action (Figure 5), from opportunity to action, with a solution that achieves five key goals:
By partnering with the right healthcare transformation company, life science companies gain two key capabilities:
While the life science industry is accustomed to utilizing data for certain insights, its next step is to scale those insights into actions—similar to how regulators and payers have realized the value of extended RWD for key decisions concerning regulatory approvals and reimbursements. By partnering with organizations committed to healthcare transformation and leveraging extended RWD, real world insights, trusted provider networks, PHM approaches, and the definitions and real-time measurements of real-world outcomes, life science companies can achieve meaningful outcomes-driven approaches to the development, regulation, launch, reimbursement, and monitoring of new therapies.
Would you like to learn more about this topic? Here are some articles we suggest:
Would you like to use or share these concepts? Download the presentation highlighting the key main points.